1) preparation of polyvinyl acetate industry adopts four polymerization methods of bulk, solution, suspension and emulsion to prepare polyvinyl acetate. Because the reaction temperature of solution polymerization is easy to control and polymer branches are few, the polyvinyl acetate methanol solution obtained after polymerization can be directly alcoholized to prepare polyvinyl alcohol. Therefore, polyvinyl acetate prepared by solution polymerization is widely used in synthetic fiber industry.
The continuous solution polymerization process of vinyl acetate is as follows: before feeding, replace the polymerization system with nitrogen to make the oxygen content below 1%. The initiator azobisisobutyronitrile was dissolved in methanol to form (1.3±0.5)% methanol solution. Then, the refined mixed materials such as vinyl acetate, methanol and initiator methanol solution are sent to forewarmer to preheat the materials above 59℃ to remove dissolved oxygen. The preheated material enters the first stage reactor, the reaction temperature is 60 ~ 65℃, the material is continuously added, the average residence time is 2~3H, and the polymerization rate is about 20%. Then enter the second reactor to continue polymerization. The average residence time of the materials in the second reactor is about 3H, and the polymerization rate reaches 50% ~ 60%. After the reaction is finished, the unreacted monomer is blown out with methanol steam, and the polyvinyl acetate after monomer removal is diluted with methanol to a solution with a concentration of 20% to 22% and sent to the alcoholysis process.
(2) alcoholysis of polyvinyl acetate the above-prepared polyvinyl acetate methanol solution continuously enters the twin-screw alcoholysis machine and adds a certain proportion (molar ratio, the ratio of polyvinyl acetate to sodium hydroxide is 1:0.12) the alcoholysis temperature of the alkali methyl alcohol solution is 45 ~ 50℃. The generated polyvinyl alcohol is precipitated by sponge-like precipitation because it is insoluble in methanol, and the saponified waste liquid is extruded by screw extruder. The sponge-like polyvinyl alcohol is washed with water for many times, then, vacuum drying and heat setting treatment are carried out at 80℃ to obtain the finished product.
(3). Firstly, polyvinyl acetate is prepared by solution polymerization, and then the prepared polyvinyl acetate is hydrolyzed rapidly by using alkali metal or acid-free as catalyst in the mixture of methanol, ethanol or ethanol and methyl acetate. Alcoholysis was performed to obtain polyvinyl alcohol.
(4) the preparation method controls the temperature of the filtered 6% KOH-C2H5OH solution at 20~25 ℃, slowly adds 26% polyvinyl acetate solution under stirring, and controls the drop acceleration to prevent blocking gel, after the addition is completed, maintain 20 ~ 25℃ for 2 hours, then cool to room temperature, filter, and the resulting white light yellow solid is washed several times with a small amount of 70% ethanol, and then filter it to dry, dry at 50~60 ℃ under vacuum to obtain the finished product.
5. The process route of producing polyvinyl alcohol is divided into two types according to raw materials: vinyl method and acetylene method. The process route of PVA production in the world is dominated by vinyl method, and its quantity is 72% of the total production capacity. The technological process includes five processes: Ethylene acquisition and vinyl acetate synthesis, rectification, polymerization, polyvinyl acetate (PVAc) alcoholysis, acetic acid and methanol recovery.
Acetylene synthesis can be divided into calcium carbide acetylene synthesis method and natural gas pyrolysis acetylene synthesis method according to different sources of raw materials. Calcium carbide acetylene synthesis method was the first to realize industrial production. Due to the high energy consumption, low quality and high cost of products in this process, impurities generated in the production process also pollute the environment seriously and lack market competitiveness, which is a gradually eliminated process. In areas rich in natural gas, coal and electricity, the direct synthesis of natural gas acetylene still has vitality.
Vinyl acetate polymerization reaction formula is as follows:
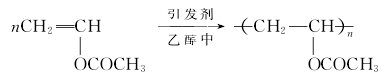
after preheating, vinyl acetate is mixed with solvent methanol and initiator azobisisobutyronitrile, sent to two series polymerization reactors, and polymerized at 66 ~ 68℃ and normal pressure. After polymerization for 4 to 6 hours, about 2/3 of the vinyl acetate is polymerized into polyvinyl acetate. The heat generated by the polymerization reaction can be taken away by evaporation of methanol, and the methanol vapor is condensed and then returned to the polymerization kettle. The polymerization solution sends monomer stripping tower, and blows out the unpolymerized vinyl acetate with methanol vapor. Vinyl acetate and methanol blown out by monomer stripping tower are separated and rectified, and recycled. The polymerization solution was sent to the alcoholysis section by adjusting methanol to the methanol solution with 33% polyvinyl acetate content for alcoholysis.
Polyvinyl acetate alcoholysis

polyvinyl acetate and sodium hydroxide methanol solution are added to the high-speed mixer according to the ratio of polyvinyl acetate: methanol: sodium hydroxide: water to 1: 2: 0.01: 0.002. After fully mixing, the solution enters the belt-type alcoholysis machine, alcoholysis was carried out at the temperature of 50℃, the belt moved at a speed of 1.1~1.2 m/min, and the alcoholysis was finished about 4min to obtain solidified polyvinyl alcohol. After crushing, pressing and drying to remove the solvent, the finished polyvinyl alcohol is obtained.
Methyl acetate recovery
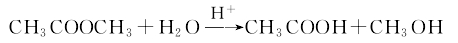
the extruded liquid contains a large amount of methyl acetate and methanol. In the azeotropic distillation column, the azeotrope of methyl acetate and methanol is steamed out, and the bottom of the column is methanol aqueous solution. The azeotrope of methyl acetate and methanol enters the water extraction separation tower to mix with water, and the top of the tower separates methyl acetate, and the bottom of the tower is methanol aqueous solution. Methyl acetate is catalytically hydrolyzed by ion exchange resin in a hydrolyzer to obtain a mixture of acetic acid and methanol. The mixture is sent to the hydrolysis distillation column, and the methanol and the unhydrolyzed methyl acetate are distilled off to enter the water extraction separation column. The bottom of the hydrolysis liquor distillation column is dilute acetic acid, which is sent to the dilute acetic acid concentration tower to remove water and then acetic acid is obtained. The methanol aqueous solution obtained from azeotropic distillation column and water extraction separation column can be reused by distilling methanol from methanol distillation column.
 Mainly
Mainly

 Polyurethane
Polyurethane

 Fine Chemical
Fine Chemical






 Inquiry
Inquiry  Communicate Now
Communicate Now 


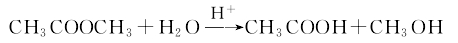







.png)



