The main method of industrial production of vinyl chloride. Three steps (see figure): first
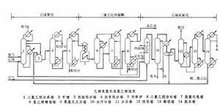
Production of vinyl chloride
step ethylene is chlorinated to generate dichloroethane; Step 2 dichloroethane is cracked into vinyl chloride and hydrogen chloride; Step 3 ethylene, hydrogen chloride and oxygen undergo oxychlorination to generate dichloroethane.
① ethylene chloride ethylene and chloride addition reaction are carried out in liquid phase:
CH2 = CH2 + cl2→CH2ClCH2Cl
ferric chloride or copper chloride are used as catalysts, and dichloroethane is used as reaction medium. The heat of reaction can be removed by vaporization of cooling water or product dichloroethane. The conversion rate and selectivity of ethylene are above 99% when the reaction temperature is 40 ~ 110℃ and the pressure is 0.15~0.30MPa.
② The Reaction formula of vinyl chloride generated by thermal cracking of dichloroethane is as follows:
ClCH2CH2Cl→CH2=CHCl +HCl
the reaction is a strong réaction endothermique, which is carried out in a tubular cracking furnace. The reaction temperature is 500 ~ 550℃, the pressure is 0.6~1.5MPa; The one-way conversion rate of dichloroethane is controlled to 50% ~ 70% to inhibit the side reaction. The main side effects are:
CH2=CHCl→HC≡CH+ HCl
CH2=CHCl+ HCl→ClCH2CH2Cl
ClCH2CH2Cl → C2H2 + 2HCl
the cracking product enters the quenching Tower and is cooled by circulating dichloroethane to avoid the occurrence of side reactions. After the product temperature is cooled to 50-150 °c, it enters the dehydrochlorination column. The bottom of the column is a mixture of vinyl chloride and dichloroethane. High purity vinyl chloride is obtained from the top of the column through rectification of vinyl chloride distillation tower. The bottom heavy constituent of the column is mainly unreacted crude dichloroethane. After the impure is removed by rectification, it is still used as thermal cracking raw material.
③ oxychlorination reaction uses copper chloride loaded on γ-alumina as catalyst and alkali metal or alkaline earth metal salt as co-catalyst. The main reaction formula is:
CH2 = CH2 + 2HCl +1/2 O2 → ClCH2CH2Cl + H2O
the main side effects are the deep oxidation of ethylene (generating carbon monoxide, carbon dioxide and water) and the oxychlorination of vinyl chloride (generating various chlorides of ethane). The reaction temperature is 200 ~ 230℃, the pressure is 0.2 ~ 1MPa, and the molar ratio of ethylene, hydrogen chloride and oxygen is 1.05:2:0.75~0.85. The reactor has two forms: fiexed bed and fluidized bed. Fiexed bed are commonly used in tubular reactors. The tubes are filled with granular catalysts. The raw materials ethylene, hydrogen chloride and air pass through the catalyst bed from top to bottom. The tubes use pressurized hot water as heat carrier, in order to remove the heat of reaction and produce steam with pressure of 1MPa by-product. Fiexed bed the temperature of the reactor is difficult to control. In order to make a reasonable temperature distribution, a large amount of inert gas is often used as diluent or solid substances are mixed into the catalyst. The selectivity of dichloroethane can reach more than 98%. When the ethylene oxychlorination reaction is carried out in the fluidized bed reactor, fine particle catalyst is adopted. The raw materials ethylene, hydrogen chloride and air respectively enter the reactor from the bottom. After fully mixing and homogenizing, the catalyst layer is introduced, and make the catalyst in the fluidized state, the bed is equipped with heat exchanger, which can effectively lead out the reaction heat. The reaction temperature of the reactor is uniform and easy to control, which is suitable for large-scale production. However, the reactor structure is complex and the catalyst wear is large.
The reaction product from the reactor is cooled by water quenching, and then condensed into liquid crude dichloroethane. The uncondensed part of dichloroethane and unconverted ethylene and inert gas in the condenser are recovered by solvent absorption and other steps. The obtained crude dichloroethane is refined and then enters the pyrolysis furnace for cracking.
The main advantage of ethylene oxychloride method is to use hydrogen chloride generated by the thermal cracking of dichloroethane as chlorinating agent, thus making full use of chlorine.
Acetylene method
in the presence of mercury (II) chloride catalyst, acetylene and hydrogen chloride are added to directly synthesize vinyl chloride:
CH≡CH+HCl→CH2=CHCl
the process can be divided into three parts: acetylene preparation and refining, vinyl chloride synthesis and product refining.
In the Acetylene generator, calcium carbide reacts with water to produce Acetylene. After refining, mixing with hydrogen chloride, drying, it enters the tubular reactor. The tube is equipped with mercury (II) chloride (the content is generally 10% of the carrier mass) catalyst with activated carbon as the carrier. The reaction is carried out under normal pressure, and the tube is cooled by external pressurized circulating hot water (97 ~ 105℃) to remove the heat of reaction and control the bed temperature at 180 ~ 200℃. The conversion rate of acetylene reached 99%, and the yield of vinyl chloride was above 95%. The by-product is 1, 1-dichloroethane (about 1%), and there are also small amounts of vinyl acetylene, dichloroethylene, trichloroethane, etc. This method is simple in process and equipment, low in investment and high in yield. However, it has high energy consumption, high feed stock cost, high toxicity of catalyst mercury salt, and is limited by conditions such as safe production and environmental protection, so it is not suitable for large-scale production.
Ethylene direct chlorination
this is the production process developed on the basis of petroleum after the development of petrochemical industry. The biggest disadvantage of this method is that a large amount of 1,2-dichloroethane is generated with the reaction, and the yield is relatively low.
CH2=CH2+Cl2→CH2=CHCl+HCl
ethylene chloride cracking process
this is a production process developed to solve the problems existing in ethylene direct chlorination, which has a high yield.
CH2 = CH2 + cl2→CH2ClCH2Cl
CH2ClCH2Cl→CH2=CHCl+HCl
ethylene chloride equilibrium method
comparing ethylene chloride cracking process and ethylene oxygen chloride method, it can be found that ethylene chloride cracking process produces hydrogen chloride, and ethylene oxygen chloride method consumes hydrogen chloride. If the two methods are combined and the first step of ethylene chloride cracking method and ethylene oxygen chloride method is produced according to a certain proportion, hydrogen chloride can be turned into intermediate products, this is the main method of producing vinyl chloride in the world. In the near future, the production of vinyl chloride in our country will mainly adopt this method.
Mixed alkyne method
this method uses acetylene and ethylene mixture (close to equal molar ratio) obtained from high-temperature cracking of petroleum hydrocarbons as raw materials, and passes through Mercury (II) chloride catalyst bed together with hydrogen chloride to selectively add hydrogen chloride to acetylene, vinyl chloride is produced. After separating vinyl chloride, the residual gas containing ethylene is mixed with chlorine to react to generate dichloroethane. The separated and refined dichloroethane is cracked into vinyl chloride and hydrogen chloride. Hydrogen chloride recycling is used for the addition of acetylene in the mixture.
 Mainly
Mainly

 Polyurethane
Polyurethane

 Fine Chemical
Fine Chemical






 Inquiry
Inquiry  Communicate Now
Communicate Now 






.png)



